What Is Likely to Be Observed for a Positive Test for the Schiffs Test?
| Classification | Colorimetric method |
|---|---|
| Analytes | Aldehydes |
-
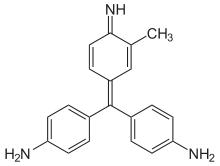
The structure of the fuchsin dye.
The Schiff examination is an early on organic chemical science named reaction developed past Hugo Schiff,[1] and is a relatively general chemical test for detection of many organic aldehydes that has also found use in the staining of biological tissues.[2] The Schiff reagent is the reaction product of a dye formulation such every bit fuchsin and sodium bisulfite; pararosaniline (which lacks an aromatic methyl group) and new fuchsin (which is uniformly mono-methylated ortho to the dye'south amine functionalities) are not dye alternatives with comparable detection chemistry.
In its employ equally a qualitative test for aldehydes, the unknown sample is added to the decolorized Schiff reagent; when aldehyde is present a characteristic magenta colour develops. Schiff-blazon reagents are used for various biological tissue staining methods, e.g. Feulgen stain and periodic acid-Schiff stain. Human peel besides contains aldehyde functional groups in the termini of saccharides and so is stained as well.
Machinery [edit]
Fuchsin solutions appear colored due to the visible wavelength absorbance of its cardinal quinoid structure—run across besides for example viologen —but are "decolorized" upon sulfonation of the dye at its central carbon atom past sulfurous acrid or its cohabit base, bisulfite. This reaction disrupts the otherwise favored delocalized extended pi-electron organisation and resonance in the parent molecule.[three]

The structure of the "decolorized" Schiff reagent.
The further reaction of the Schiff reagent with aldehydes is complex with several research groups reporting multiple reaction products with model compounds. In the currently accepted mechanism, the pararosaniline and bisulfite combine to yield the "decolorized" adduct with sulfonation at the central carbon as described and shown. The free, uncharged aromatic amine groups then react with the aldehyde being tested to form two aldimine groups; these groups take likewise been named for their discoverer as Schiff bases (azomethines), with the usual carbinolamine (hemiaminal) intermediate being formed and dehydrated en road to the Schiff base. These electrophilic aldimine groups so react with further bisulfite, and the Ar-NH-CH(R)-SO3 − product (and other resonance-stabilized species in equilibrium with the production) give rise to the magenta color of a positive test.[four] Prior germination of classical bisulfite adducts of the tested aldehyde may, when the adducts are stable, requite ascent to false negative tests such as in the case of testing for the aldehydic terminus of glucose.[4] Schiff's reagent on reaction with Acetaldehyde gives pinkish colour.
Such an imine-mediated machinery was first proposed by Paul Rumpf (1908–1999) in 1935,[5] and experimental testify was provided by Hardonk and van Duijn in 1964.[6] In 1980, Robins, Abrams and Pincock provided substantial NMR evidence for the mechanism, leading to its general acceptance.[vii] Stoward had examined the mechanism in 1966 and, on the whole, considered this mechanism to be correct.[viii]
A 2d, earlier mechanism continues to announced in the literature.[9] The machinery was proposed in 1921 by the eminent German organic chemist Heinrich Wieland and his student Georg Scheuing (1895–1949).[10] [11] Bisulphite was believed to react with the available aromatic amine functional groups to form Northward-sulfinic acid groups, Ar-NH-SOiiH, followed by reaction with aldehyde to form sulfonamides, Ar-NH-SO2CH(OH)-R. The 1980 NMR data that immune visualization of intermediates does not support this mechanism or the sulfonamides as the chromogenic product.[7]
See besides [edit]
- Tollens' reagent
- Fehling'south reagent
- 2,four-Dinitrophenylhydrazine
- Fehling's solution
- Barfoed's test
- Benedict'due south reagent
References [edit]
- ^ See:
- Schiff, Hugo (1866) "Eine neue Reihe organischer Diaminen. Zeite Abteilung." (A new series of organic diamines. 2d function.), Justus Liebigs Annalen der Chemie, 140 : 92–137. [in German language] From p. 132: "Das Rosanilinsulfit verbindet sich nicht directly mit den Aldehyden. Schüttelt man dice rothe Lösung des krystallisirten neutralen Sulfits oder auch die nach obiger Weise bereitete leukanilinhaltige gelbe Lösung mit irgend einem flüssigen Aldehyd, so erhält homo sogleich eine rothe Lösung, und diese Farbe verwandelt sich allmählich in ein je nach dem angewandten Aldehyd helleres oder dunkleres Violettblau." (Rosaniline [i.e., fuchsine] sulfite does not combine directly with aldehydes. If one shakes, with any liquid aldehyde, a ruby-red solution of the crystallized neutral sulfite [of fuchsine] or even the xanthous solution which was prepared in the above-mentioned mode and which contains leukaniline [i.eastward., fuchsine treated with aqueous sulfurous acid], then ane obtains at in one case a red solution, and this color is transformed into a lighter or darker violet blue, depending on the aldehyde that'due south used.)
- See also: Schiff, Ugo (1866) "Sopra una nova serie di basi organiche" (On a new serial of organic bases), Giornale di scienze naturali ed economiche, ii : 201–257. [in Italian] From p. 253: "Il solfito di rosanilina not si combina direttamente colle aldeidi. Quando si agita una soluzione solforosa di idrato o di acetato di rosanilina con united nations'aldeide qualunque, allora la soluzione gialla ássume subito un colore rosso che poco a poco si trasforma in un bel violetto più or meno intenso a secondo fifty'aldeide applicata." (Rosaniline sulphite does not combine directly with aldehydes. When a sulfurous solution of rosaniline hydrate or rosaniline acetate is shaken with any aldehyde, then the yellowish solution immediately becomes a red color that little by little transforms into a fine violet, [which is] more or less intense according to the aldehyde [that'due south] used.)
- Run across too: Schiff, Hugo (1867) "Dérivés de la rosaniline" (Derivatives of rosanaline [i.e., fuchsine]), Comptes rendus, 64 : 182–184. [in French] From p. 182: " … si l'on agite une solution sulfureuse diluée, soit de sulfite, soit de tout autre sel de rosaniline, avec quelques gouttes d'united nations aldéhyde, alors il se dégage de 50'acide sulfureux, la solution se colore d'abord en rouge, puis en violet, et peu à peu il se forme un précipité constitué de petites écailles cristallines d'un violet cuivré." ( … if one shakes a dilute sulfurous solution either of the sulphite or whatever other salt of rosaniline [i.due east., fuchsine] with a few drops of an aldehyde, then sulphurous acrid is evolved; the solution is outset colored red, and so violet, and gradually a precipitate is formed consisting of modest crystalline flakes of a coppery violet.)
- ^ Histology, cell and tissue biology 5th Ed., 1983 ISBN 0-333-35406-0
- ^ Juan Carlos Stockert, Alfonso Blázquez-Castro (2017). Fluorescence Microscopy in Life Sciences. Bentham Science Publishers. ISBN978-1-68108-519-7 . Retrieved 17 December 2017.
- ^ a b "Schiffsche Probe" (Schiff examination), University of Bayreuth, Bavaria, Germany, accessed 8 March 2013. [in German]
- ^ Rumpf, P. (1935) "Recherches physico-chimiques sur la réaction colorée des aldéhydes dite "réaction de Schiff" " (Concrete-chemical investigations of the colour reaction of aldehydes, called the "Schiff reaction"), Annales de Chimie, 11th series, 3 : 327–442. [in French]
- ^ Hardonk, M.J. & Van Duijn, P. (1964) "The mechanism of the Schiff reaction as studied with histochemical model systems," Journal of Histochemistry & Cytochemistry, 12 (10) : 748–751.
- ^ a b Robins, J.H., Abrams, Thou.D. & Pincock, J.A. (1980) "The construction of Schiff reagent aldehyde adducts and the mechanism of the Schiff reaction as determined by nuclear magnetic resonance spectroscopy," Canadian Journal of Chemistry, 58 (iv) : 339–347.
- ^ Stoward, P.J. (1966) "Some comments on the mechanism of the Schiff reaction," Journal of Histochemistry & Cytochemistry, xiv (9) : 681–683.
- ^ Histochemistry, theoretical and applied quaternary Ed. 1985 ISBN 0-443-02997-0 Annotation: the depiction of the sulfonic acid mechanism in this edition contains an error as the aldehyde R group is bonded to nitrogen and not to its carbon neighbor
- ^ Wieland, Heinrich ; Scheuing, Georg (1921) "Die Fuchsin-schweflige Säure und ihre Farbreaktion mit Aldehyden" (Fuchsine-sulfurous acid and its colored reaction with aldehydes), Berichte der deutschen chemischen Gesellschaft, 54 (x) : 2527–2555. [in German]
- ^ Puchtler, Holde; Meloan, Susan N.; Brewton, Barbara R. (1975) "On the history of basic fuchsin and aldehyde-Schiff reactions from 1862 to 1935," Histochemistry, 41 (3) : 185–194.
External links [edit]
- What is Schiff's reagent?
Source: https://en.wikipedia.org/wiki/Schiff_test
0 Response to "What Is Likely to Be Observed for a Positive Test for the Schiffs Test?"
Post a Comment